Inside the STOPSTORM Project
Project Overview
STOPSTORM is the acronym for Standardized Treatment and Outcome Platform for Stereotactic Therapy Of Re-entry tachycardia by a Multidisciplinary consortium. It is a European collaborative research project aimed at evaluating, standardizing, and clinically validating Stereotactic Arrhythmia Radioablation (STAR) as a non-invasive treatment option for patients with therapy-refractory ventricular tachycardia (VT).
STOPSTORM is funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 945119. Full project details are available on the official CORDIS project page.
Medical Background
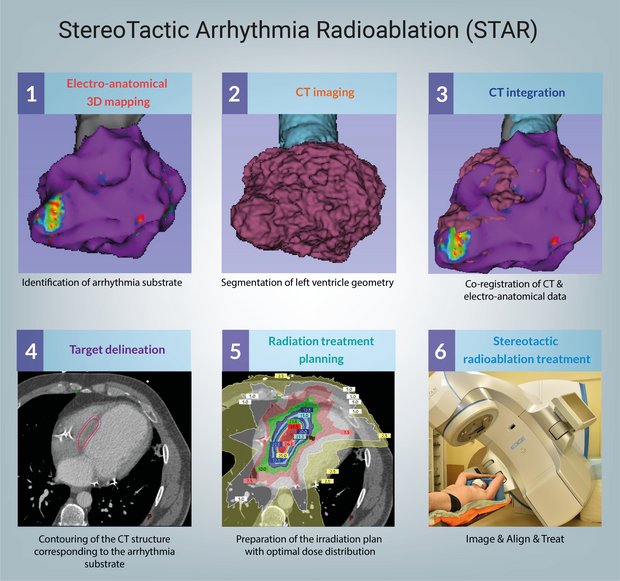
Patients suffering from ventricular tachycardia are typically treated with antiarrhythmic drugs, catheter ablation, and implantable cardioverter-defibrillators (ICDs) to reduce arrhythmia burden and prevent sudden cardiac death. However, treatment failures and VT recurrence following catheter ablation remain significant concerns, particularly as the number of affected patients continues to grow.
STAR—Stereotactic Arrhythmia Radioablation—is an emerging treatment modality that involves delivering highly focused radiation to the arrhythmogenic substrate within the myocardium. Clinically introduced in 2012, STAR has been investigated in several preclinical and clinical studies, with promising results showing a substantial reduction in arrhythmic burden in otherwise untreatable VT patients. While clinical experience with STAR is still limited, it is rapidly expanding through ongoing single-center and multicenter trials at pioneering institutions.
Common inclusion criteria for STAR typically involve the presence of structural heart disease and recurrent symptomatic monomorphic VT, despite optimized antiarrhythmic therapy, ICD intervention, and prior (failed) catheter ablation. However, criteria may vary across centers and national regulatory frameworks.
Details on STAR are summarized in several well-written pre-clinical, clinical and technical reviews which you can find here: STOPSTORM_Community@Zenodo
STOPSTORM Objectives
The primary aim of the STOPSTORM project is to harmonize and coordinate European efforts in STAR research by consolidating all ongoing trials and patient data within a unified registry platform. The project seeks to enable consistent treatment protocols, improved patient selection, standardized imaging and target delineation, and evidence-based outcome assessment.
If you are interested in referring a patient or participating in the STOPSTORM registry, please contact our project management team at stopstorm(at)email.uni-kiel.de, and they will connect you with your nearest participating center.
Project Structure
STOPSTORM is organized into nine Work Packages (WPs), each addressing a specific aspect of the projectEach Work Package is led by a dedicated expert team, ensuring high scientific quality, transparency, and multidisciplinary integration across the consortium.
Lead: Universitätsspital Zürich, Switzerland
Objectives
• To establish a clinical database and implementation of a PACS infrastructure for image, RT structure, and outcome data collection of patients treated with STAR for sustained refractory VT in structural heart disease.
• To collect clinical, imaging, treatment, and outcome data of patients already treated outside of the STOPSTORM prospective cohorts.
• To analyse various clinical, treatment, and outcome parameters in order to assess overall data quality from the observational data cohort and, based on these initial experiences, define a minimum set of required parameters for the prospective cohort from WP3.
• To define a set of standardised variables to be collected prospectively from patients treated outside of the prospective validation cohort (for WP3).
• To collect data from prospective clinical trials of STAR not fulfilling the inclusion criteria of the prospective STOPSTORM validation cohort.
• To investigate patterns of care and derive minimum recommendations for safe delivery of STAR, before WP2 and WP3 data becomes available.
Lead: Leiden University Medical Center, Netherlands
Objectives:
• Develop benchmarks for invasive electro-anatomical substrate delineation.
• Develop benchmarks for non-invasive electro-anatomical substrate delineation.
• Develop benchmarks for accurate EP data transfer to the radiation planning’s system.
• Provide knowledge transfer to participating centres.
• Develop feedback structure for standardisation.
Lead: Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie – Państwowy Instytut Badawczy, Poland
Objectives:
• To prospectively collect data on safety and efficacy of STAR treatment.
• To use developed procedures of transmission of electrophysiological data from EP mapping to radiosurgery planning systems, patient setup, and management of respiratory motion during treatment delivery.
• To provide outcome measures allowing for the evaluation of data in terms of treatment safety and efficacy. The results of the analysis should allow for preparation of European guidelines for stereotactic ablation of ventricular tachycardia (STAR treatment).
Lead: University Hospital Schleswig-Holstein Kiel, Germany
Objectives:
• To establish a credentialing and audit committee for STOPSTORM.
• To develop a quality assessment programme for centres performing STAR.
• To guarantee quality assurance of clinical structure delineation, treatment planning, and treatment delivery of STAR in Europe.
• To ensure harmonisation of STAR treatment quality for the prospective registry and throughout the various clinical trials for STAR in Europe.
• To enable central review of STAR treatment in Europe.
Lead: University Medical Center Utrecht, Netherlands
Objectives:
• To conduct a multivariate and subgroup analysis that confirms the primary endpoints of the prospective STOPSTORM trial (WP3).
• To pool all observationally (WP1) and prospectively (WP3) collected data for joint analysis.
• To enable exploratory hypothesis testing on all collected datasets.
• To establish a dose-effect relationship for targets and OARs.
• To facilitate a longitudinal biomarker search on all collected physiological data.
Lead: Azienda Unita Sanitaria Locale Di Reggio Emilia, Italy
Objectives:
• To provide an ethical and legal framework for the research project.
• To translate current critical ethical and legal issues into study-specific guidelines in order to evaluate whether the study is ethically feasible and respectful of patients’ vulnerability and autonomy.
• To collect ethical evidence through empirical research on several fundamental dimensions of the doctor-patient relationship.
• To draw up guidelines for legal agreements/contracts that may be necessary for carrying out the research project.
Lead: University Hospital Schleswig-Holstein Kiel, Germany
Objectives:
• To effectively communicate the results and the progress of the WPs within the consortium.
• To harmonise protocols and workflows between centres that perform the STAR procedure.
• To effectively communicate with stakeholders to gain a deeper understanding and raise awareness.
• To manage exploitation efforts undertaken by the consortium.
Lead: University Hospital Schleswig-Holstein Kiel, Germany
Objectives:
• Ensure timely and structured execution of the project according to the work plan.
• Report project progress to the European Commission.
Lead: University Hospital Schleswig-Holstein Kiel, Germany
Objectives:
- The objective is to ensure compliance with the 'ethics requirements' set out in this work package.
The overall project coordination is currently based at the University Hospital Schleswig-Holstein (UKSH), Kiel, Germany.
Project Coordinator: PD Dr. Oliver Blanck Email Project Manager: Katharina Baumann Email
The coordination team ensures seamless management of the consortium, facilitates inter-institutional collaboration, and oversees regulatory compliance, reporting, and communication with the European Commission.
